Abstract
Background: Anti-CD19 chimeric antigen receptor (CAR19) T-cells have activity in patients with relapsed/refractory large B-cell lymphoma (rrLBCL), but over half of patients ultimately relapse. We originally applied cell-free DNA (cfDNA) analysis to a cohort (n=65) of patients receiving axicabtagene ciloleucel (axi-cel) to identify determinants of resistance and characterize molecular thresholds predictive of treatment failure. Here, we extend these analyses to an additional independent cohort (n=73) of patients to validate these thresholds and refine genomic alterations associated with resistance.
Methods: We developed a novel hybrid capture strategy targeting 187 protein coding genes, all T-cell receptor (TCR) loci, and axi-cel retroviral vector. This approach allows for simultaneous evaluation of circulating tumor-derived DNA (ctDNA), CAR19-derived cfDNA (cfCAR) and T-cell receptor rearrangements (cfTCR) from blood plasma. We applied this platform to profile serial samples from discovery (n=65) and validation (n=73) cohorts of rrLBCL patients receiving CAR19 therapy (total n=128). Pretreatment and relapse tumor tissue was also profiled when available. A previously determined optimized pretreatment (Day 0) ctDNA threshold to stratify event free survival (EFS) was revalidated in the discovery cohort using bootstrap resampling (2.5 log10 haploid genome equivalents per milliliter).
Results: The median follow-up was 36.4 months in the discovery cohort, and 12.5 months in the validation cohort. 55% (36/65) and 58% (42/73) of patients in the discovery and validation cohorts had progressed following CAR19, respectively.
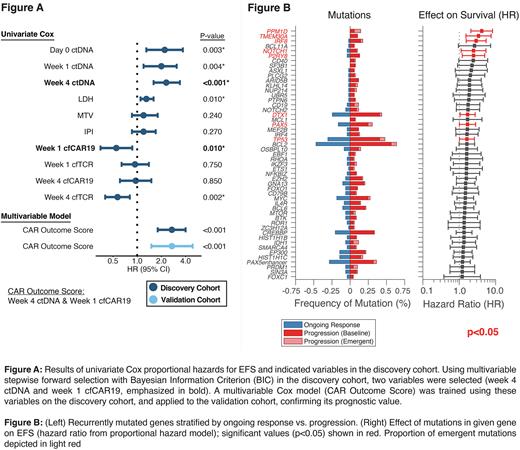
In both cohorts, patients with high pretreatment ctDNA demonstrated inferior EFS (Discovery: p<0.001, Validation, p=0.002). Similarly, a ~300-fold (2.5 log10) decrease in ctDNA levels at Day 28 (Major Molecular Response - MMR) post CAR19 infusion was identified as the optimal post-treatment change to stratify EFS, and patients in both cohorts who achieved MMR had significantly superior EFS (Discovery: p<0.001, Validation, p=0.028). When considering ctDNA, cfTCR and cfCAR as continuous variables in the discovery cohort, higher pre- and post-treatment ctDNA levels were prognostic of EFS at all assayed time-points, as were pre-treatment LDH, week 1 cfCAR19 and week 4 cfTCR levels (Fig. A). Forward stepwise multivariable regressions identified week 4 ctDNA level (HR=2.2, p<0.001) and week 1 cfCAR19 level (HR=0.6, p=0.017) as the optimal independent covariates for predicting outcomes (Fig. A). A Cox proportional hazards model incorporating these two variables was trained on the discovery cohort (CAR Outcome Score) and was validated to be prognostic for outcomes in the validation cohort (HR=2.7, p<0.001).
Genomic alterations in several genes were significantly associated with inferior outcomes in both cohorts, including PAX5, IRF8, TMEM30A, PPM1D and TP53 (Fig. B). Additional, non-stereotyped, emergent alterations were noted in relapsing patients in other genes associated with immunological escape, including mutations in CD19 and amplifications in the PD-L1 locus. CIBERSORTx was used to characterize intratumoral cell type composition, which was found to be influenced by tumor genotypes. Further, CAR19 persistence in tumors at relapse was associated with specific changes in the tumor microenvironment, including regulatory T-cell expansion and B-cell depletion.
Conclusions: Pre-treatment and dynamic ctDNA levels predict treatment failure of LBCL after CAR19 therapy, and a novel CAR outcome score incorporating week 4 ctDNA and week 1 cfCAR19 levels is strongly predictive of outcome. Genomic determinants of resistance to CAR19 therapy are diverse, including recurrent mutations in genes controlling B-cell identity (IRF8, PAX5), and those influencing the composition of the tumor microenvironment and heterotypic cell-cell interactions (TMEM30A). Additional immunological escape mechanisms arise under selective pressure from CAR19 cells.
Disclosures
Kurtz:Foresight Diagnostics: Consultancy, Current equity holder in private company, Patents & Royalties; Adaptive Biotechnologies: Consultancy; Roche: Consultancy; Genentech: Consultancy. Alig:Takeda Pharmaceuticals: Consultancy. Frank:Roche/Genentech - Wife: Current equity holder in private company, Current holder of stock options in a privately-held company; Allogene Therapeutics: Research Funding; Adaptive Biotechnologies: Consultancy, Honoraria, Research Funding; Kite/Gilead: Honoraria, Research Funding. Shahrokh Esfahani:Foresight Diagnostics: Consultancy. Beygi:Kite-a Gilead company: Current Employment, Current equity holder in publicly-traded company. Westin:Iksuda: Consultancy; Calithera: Consultancy, Research Funding; MonteRosa: Consultancy; ADC Therapeutics: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; MorphoSys/Incyte Corporation: Consultancy, Research Funding; Genentech/Roche: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; Kite, a Gilead Company: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Merck: Consultancy; Abbvie/GenMab: Consultancy; SeaGen: Consultancy. Khodadoust:CRISPR Therapeutics: Research Funding; Daiichi Sankyo: Membership on an entity's Board of Directors or advisory committees; Myeloid Therapeutics: Membership on an entity's Board of Directors or advisory committees; Nutcracker Therapeutics: Research Funding. Majzner:Syncopation: Consultancy, Divested equity in a private or publicly-traded company in the past 24 months; Link Cell Therapies: Consultancy, Divested equity in a private or publicly-traded company in the past 24 months; Lyell Immunophama: Consultancy, Divested equity in a private or publicly-traded company in the past 24 months; Innervate Radiopharmaceutics: Consultancy, Divested equity in a private or publicly-traded company in the past 24 months; Immunai: Consultancy; NKARTA: Consultancy; Aptorum Group: Consultancy, Divested equity in a private or publicly-traded company in the past 24 months; Fate-DSMB: Consultancy. Mackall:BMS: Consultancy; Medimmune Tech: Consultancy; Link: Consultancy, Divested equity in a private or publicly-traded company in the past 24 months; Ensoma: Divested equity in a private or publicly-traded company in the past 24 months; Syncopation: Consultancy, Divested equity in a private or publicly-traded company in the past 24 months; Nektar: Consultancy; Mammoth: Divested equity in a private or publicly-traded company in the past 24 months; Lyell Pharmaceuticals: Consultancy, Divested equity in a private or publicly-traded company in the past 24 months; Apricity: Consultancy, Divested equity in a private or publicly-traded company in the past 24 months; Immatics: Consultancy; GSK: Consultancy. Diehn:Foresight Diagnostics: Consultancy, Current equity holder in private company. Miklos:Bristol Meyers Squibb: Consultancy; Novartis: Consultancy; Pharmacyclics: Patents & Royalties: cGVHD Ibrutinib patent ; Janssen: Consultancy, Honoraria; Adaptive Biotech: Consultancy; Fosun Kite: Consultancy, Honoraria; Kite, a Gilead Company: Research Funding; Allogene: Research Funding. Alizadeh:Adaptive Biotechnologies: Consultancy; Genentech: Consultancy; Cibermed Inc: Consultancy, Current equity holder in private company, Patents & Royalties; Foresight Diagnostics: Consultancy, Current equity holder in private company, Patents & Royalties; Roche: Consultancy; BMS: Consultancy, Research Funding; Syncopation: Current equity holder in private company, Patents & Royalties; Karyopharm: Consultancy; Gilead: Consultancy, Divested equity in a private or publicly-traded company in the past 24 months, Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal